Anakinra Covid-19, Study Backs Cytokine Targeting For Covid 19 Tx Medpage Today
Severe COVID-19 cases have a detrimental hyper-inflammatory host response and different cytokine-blocking biologic agents were explored to improve outcomes. Confirmation of efficacy will require controlled trials.

Early Identification Of Covid 19 Cytokine Storm And Treatment With Anakinra Or Tocilizumab International Journal Of Infectious Diseases
We aimed to assess the effect of anakinra treatment on mortality in patients admitted.
Anakinra covid-19. On the clinical use of the interleukin-1 IL-1 receptor antagonist anakinra to treat patients with COVID-19 is very interesting. The SAVE-MORE double-blind randomized controlled trial evaluated the efficacy and safety of anakinra an IL-1αβ inhibitor in 594 patients with COVID-19 at risk of progressing to respiratory failure as identified by plasma suPAR 6 ng ml-1 859 n 510 of whom were receiving dexamethasone. Interleukin IL-1 receptor anakinra has been used as an off-label agent for treatment of COVID-19 during the COVID-19 pandemic.
Anakinra a recombinant interleukin IL-1 receptor antagonist may have a beneficial effect in patients with severe COVID-19 with a significant inflammatory response. Clinical Trial of the Use of Anakinra in Cytokine Storm Syndrome Secondary to Covid-19 ANA-COVID-GEAS ANA-COVID-GEAS The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Efficacy of Intravenous Anakinra and Ruxolitinib During COVID-19 Inflammation JAKINCOV The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
Anakinra could be a safe anti-inflammatory treatment option to reduce the mortality risk in patients admitted to hospital with moderate to severe COVID-19 pneumonia especially in the presence of signs of hyperinflammation such as CRP concentrations higher than 100 mgL. A pathological dysregulated immune response ie hyperinflammation is a recognised complication of COVID-191 The protype hyperinflammatory syndrome secondary to infection is secondary haemophagocytic lymphohistiocytosis sHLH but the dominant hyperinflammatory phenotype in people with severe COVID-19-associated pneumonia is not that of sHLH and is poorly. Available evidence shows that treatment with anakinra reduces both the need for invasive mechanical ventilation and mortality risk of hospitalized non-intubated patients with COVID-19 without increasing the risk of adverse events.
Reports during the early COVID-19 pandemic. Patients in the interventional arm received subcutaneous anakinra 100 mg twice daily for 3 days. In the investigators case-series using anakinra in patients with COVID-19 showed promising in preventing the need for mechanical ventilation and mortality subsequently.
Anakinra might improve the prognosis of patients with moderate to severe COVID-19 ie patients requiring oxygen supplementation but not yet receiving organ support. We investigated the effects of anakinra on inflammatory parameters and clinical outcomes in critically ill mechanically ventilated COVID-19 patients with clinical features of hyperinflammation. Listing a study does not mean it has been evaluated by the US.
Considering Russell and colleagues3 comments about potential harm of corticosteroid use in patients with Covid-19 infection the patient was commenced on treatment with the selective IL-1 receptor antagonist drug Anakinra and a two day course of intravenous immunoglobulin. Listing a study does not mean it has been evaluated by the US. A prospective open-label interventional study in adults hospitalized with severe COVID-19 pneumonia was conducted.
The aim of this study was to evaluate the efficacy of anakinra in patients who were admitted to hospital for severe COVID-19 pneumonia requiring oxygen therapy. Anakinra a recombinant interleukin-1 receptor antagonist is known to be effective in several hyperinflammatory diseases. Anakinra use in COVID-19 has been reported in several retrospective and prospective studies as well as in one randomized controlled trial 51415161718192021.
Anakinra reduced both need for invasive mechanical ventilation in the ICU and mortality among patients with severe forms of COVID-19 without serious side-effects. Anakinras ability to inhibit both IL-1 subtypes and short half-life makes it favorable to some experts. The main hypothesis of the study was based on hyperinflammation caused by an increase in proinflammatory cytokines such as IL-1β IL-6 and tumour necrosis.
Anakinra blocks the activity of both IL-1α and IL1β and is approved for different autoinflammatory disorders but it is used off-label for conditions characterized by an excess of cytokine production. The patients will receive intraveinous injection of Anakinra 400mg from Day 1 to Day 3 two injections of 100 mg each 12 hours and 200mg the remaining 7 days. At day 28 the adjusted proportional odds of.
Anakinra for severe forms of COVID-19. Treatment with anakinra may reduce mortality risk in patients hospitalized with moderate to severe COVID-19 pneumonia according to findings from a systematic review published in Lancet Rheumatology. Confirmation of efficacy and safety requires randomized placebo-contr.
Anakinra plus Optimized Standard of Care oSOC on the condition of patients with COVID-19 infection and worsening respiratory symptoms. Anakinra treatment of COVID-19 patients admitted with LRTI and suPAR concentrations greater or equal than 6 μgl was associated with a relative decrease of the incidence of SRF by 70. However its exact benefits for patients with moderate to.
However additional trials are needed to better understand the role of IL-1 receptor antagonism in COVID-19 according to a commentary published in Lancet Rheumatology. Anakinra-treated patients who were eventually admitted to the ICU had a shorter stay than those who did not receive anakinra.

Il 1 Receptor Antagonist Anakinra In The Treatment Of Covid 19 Acute Respiratory Distress Syndrome A Retrospective Observational Study The Journal Of Immunology

Frontiers Controlling Cytokine Storm Is Vital In Covid 19 Immunology
Biocentury A Mechanistic View Of Four Compounds That Disrupt Interleukin Pathways To Treat Severe Covid 19

Anakinra Reduces Ventilation Need Mortality In Covid 19

Anakinra In Hospitalized Patients With Severe Covid 19 Pneumonia Requiring Oxygen Therapy Results Of A Prospective Open Label Interventional Study International Journal Of Infectious Diseases

Interleukin 1 Receptor Antagonist Anakinra In Association With Remdesivir In Severe Covid 19 A Case Report International Journal Of Infectious Diseases

Favorable Anakinra Responses In Severe Covid 19 Patients With Secondary Hemophagocytic Lymphohistiocytosis Sciencedirect
Plos One Glucocorticoids With Low Dose Anti Il1 Anakinra Rescue In Severe Non Icu Covid 19 Infection A Cohort Study
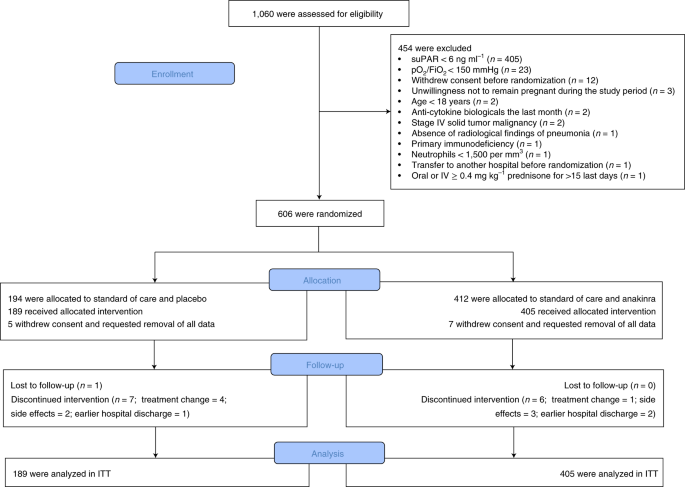
Early Treatment Of Covid 19 With Anakinra Guided By Soluble Urokinase Plasminogen Receptor Plasma Levels A Double Blind Randomized Controlled Phase 3 Trial Nature Medicine
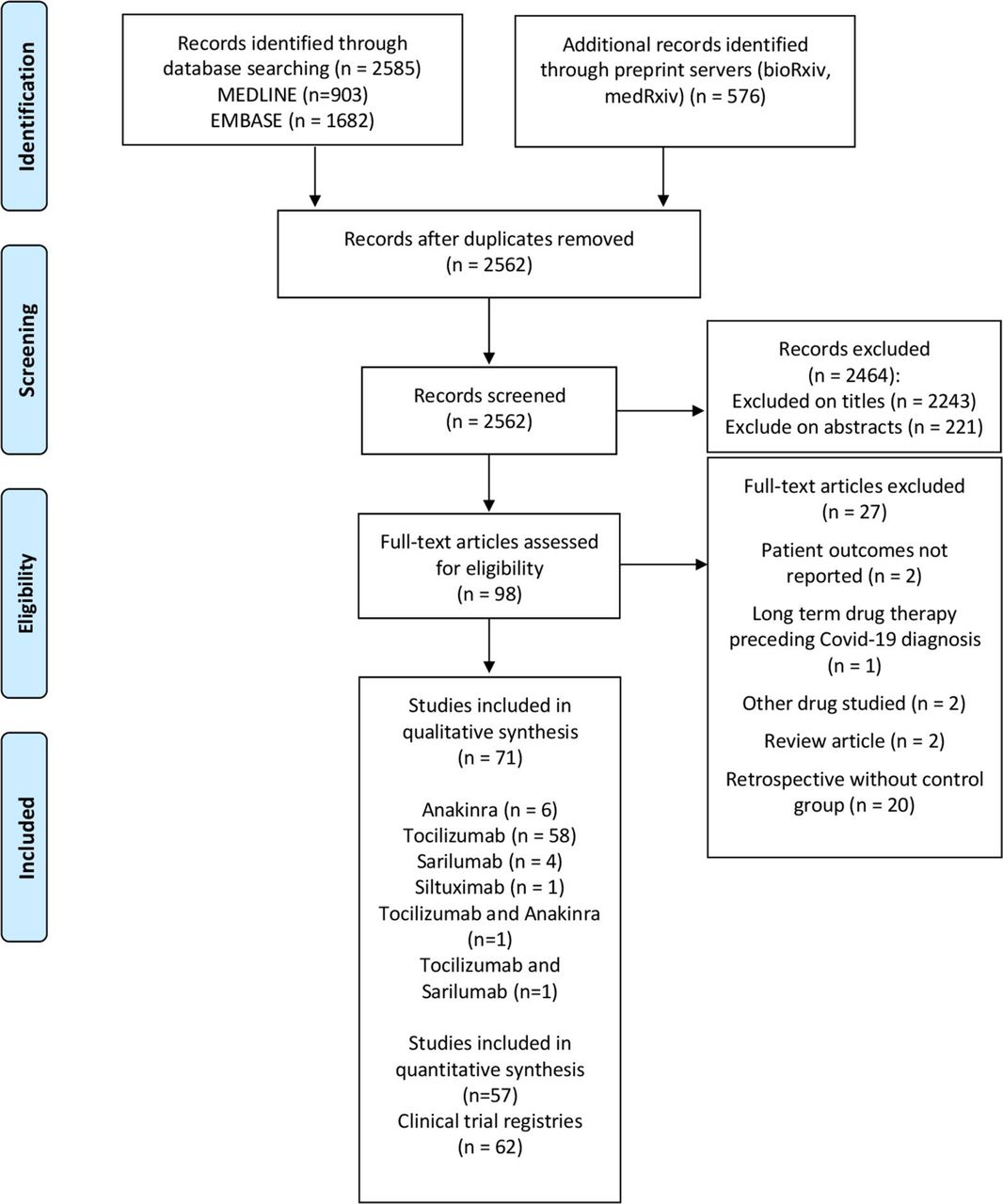
Systematic Review And Meta Analysis Of Anakinra Sarilumab Siltuximab And Tocilizumab For Covid 19 Thorax
Biocentury A Mechanistic View Of Four Compounds That Disrupt Interleukin Pathways To Treat Severe Covid 19
World Academy Of Science Engineering And Technology Linkedin

Proposal For The Use Of Anakinra In Acute Respiratory Distress Secondary To Covid 19 Sciencedirect

Study Backs Cytokine Targeting For Covid 19 Tx Medpage Today

Greek Study Suggests Mortality Benefit For Kineret In Covid 19 2021 05 20 Bioworld

Novel Paediatric Presentation Of Covid 19 With Ards And Cytokine Storm Syndrome Without Respiratory Symptoms The Lancet Rheumatology

Anakinra For Severe Forms Of Covid 19 A Cohort Study The Lancet Rheumatology

Interleukin 1 Blockade With High Dose Anakinra In Patients With Covid 19 Acute Respiratory Distress Syndrome And Hyperinflammation A Retrospective Cohort Study The Lancet Rheumatology



