Anakinra Covid 19 Treatment | Infection Chemotherapy
In the SAVE-MORE trial anakinra plus standard-of-care SOC prevented either death or progression to severe respiratory failure whilst also increasing the number of patients. Researchers found more than 70 percent of seriously ill COVID-19 patients treated with anakinra sold under the brand name Kineret showed improvements in breathing and reduced.

The Pathogenesis And Treatment Of The Cytokine Storm In Covid 19 Journal Of Infection
A drug traditionally used in the treatment of arthritis anakinra may possess benefits in the treatment of severely ill COVID-19 patients according to a new Italian study.

Anakinra covid 19 treatment. However additional trials are needed to better understand the role of IL-1 receptor antagonism in COVID-19 according to a commentary published in Lancet Rheumatology. Sonmez et al We believe that Anakinra may have a role in patients who manifest MAS due. Examined various markers of the immune response and discovered that anakinra was able to improve immune function protecting a significant number of patients from going on a.
Anakinra a recombinant interleukin IL-1 receptor antagonist may have a beneficial effect in patients with severe COVID-19 with a significant inflammatory response. Anakinra is a recombinant IL-1 receptor antagonist which is routinely used for rheumatoid arthritis and has also been used for treatment of macrophage activation syndrome. 20 sor Data was collected from 69 patients with severe COVID-19 pneumonia treated.
The main hypothesis of the study was based on hyperinflammation caused by an increase in proinflammatory cytokines such as IL-1β IL-6 and tumour necrosis factor TNF triggered by SARS-CoV-2. Therefore we aimed to assess the off-label use of anakinra in patients who were admitted to hospital for severe forms of COVID-19 with symptoms indicative of worsening respiratory function. Anakinras ability to inhibit both IL-1 subtypes and short half-life makes it favorable to some experts.
A double-blind randomized controlled phase 3 trial. EMA has started evaluating an application to extend the use of Kineret anakinra to include treatment of coronavirus disease 2019 COVID-19 in adult patients with pneumonia who are at risk of developing severe respiratory failure inability of the lungs to work properly. According to new data the early and targeted use of Kineret anakinra in hospitalised moderate to severe COVID-19 pneumonia patients with poor prognosis improved overall clinical outcomes by 64 percent.
On the clinical use of the interleukin-1 IL-1 receptor antagonist anakinra to treat patients with COVID-19 is very interesting. 12 13 14 15 In most studies anakinra was administered on an off-label basis in patients with clinical or laboratory signs of hyperinflammation or both or in the context of. Case series of a small number of patients with moderate or severe COVID-19 treated with anakinra have been published during the early period of the pandemic suggesting promising results and paving the way for additional larger studies.
At day 28 the adjusted proportional odds of having a worse clinical status assessed by the 11-point World Health Organization Clinical Progression Scale WHO-CPS with anakinra. The SAVE-MORE double-blind randomized controlled trial evaluated the efficacy and safety of anakinra an IL-1αβ inhibitor in 594 patients with COVID-19 at risk of progressing to respiratory failure as identified by plasma suPAR 6 ng ml-1 859 n 510 of whom were receiving dexamethasone. Treatment with anakinra may reduce mortality risk in patients hospitalized with moderate to severe COVID-19 pneumonia according to findings from a systematic review published in Lancet Rheumatology.
The findings show that treating COVID-19 patients with an injection of 100 milligrams of anakinra for ten days may be an effective approach because the drug combats inflammation. There are currently no treatments directed at halting the cytokine storm and acute lung injury to stop the progression from manageable hypoxia to frank respiratory failure and ARDS in patients with COVID-19 infection. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels.
In the investigators case-series using anakinra in patients with COVID-19 showed promising in preventing the need for mechanical ventilation and mortality subsequently. These data led us to hypothesise that anakinra could represent an efficient treatment for severe forms of COVID-19 predominantly involving the inflammasome pathway. However its exact benefits for patients with moderate to.
Here we report results of an open-label treatment with a combination of an IL-6 receptor blocker tocilizumab and an IL-1 receptor antagonist anakinra in patients with early up to 10 days since symptom onset severe COVID-19 pneumonia with evidence of cytokine release. Interleukin IL-1 receptor anakinra has been used as an off-label agent for treatment of COVID-19 during the COVID-19 pandemic. Kineret is an immunosuppressant a medicine that reduces the activity of.

Early Identification Of Covid 19 Cytokine Storm And Treatment With Anakinra Or Tocilizumab International Journal Of Infectious Diseases

Study Backs Cytokine Targeting For Covid 19 Tx Medpage Today

Anakinra For Severe Forms Of Covid 19 A Cohort Study The Lancet Rheumatology

Interleukin 1 Blockade With High Dose Anakinra In Patients With Covid 19 Acute Respiratory Distress Syndrome And Hyperinflammation A Retrospective Cohort Study The Lancet Rheumatology
Plos One Glucocorticoids With Low Dose Anti Il1 Anakinra Rescue In Severe Non Icu Covid 19 Infection A Cohort Study
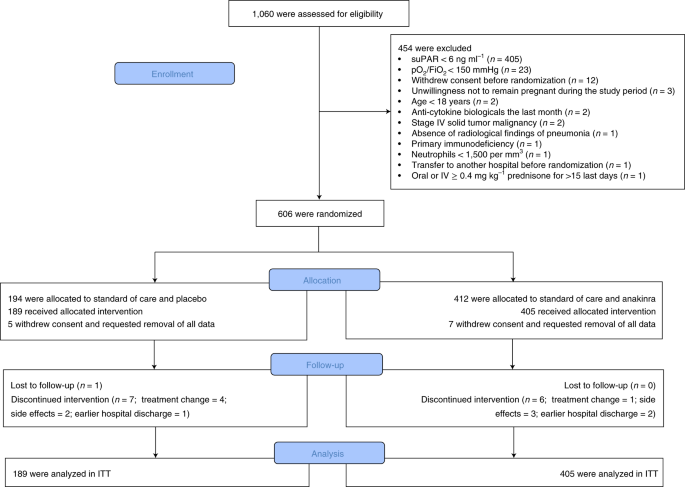
Early Treatment Of Covid 19 With Anakinra Guided By Soluble Urokinase Plasminogen Receptor Plasma Levels A Double Blind Randomized Controlled Phase 3 Trial Nature Medicine

Safety Perspectives On Presently Considered Drugs For The Treatment Of Covid 19 Penman 2020 British Journal Of Pharmacology Wiley Online Library

Anakinra In Hospitalized Patients With Severe Covid 19 Pneumonia Requiring Oxygen Therapy Results Of A Prospective Open Label Interventional Study International Journal Of Infectious Diseases

Favorable Anakinra Responses In Severe Covid 19 Patients With Secondary Hemophagocytic Lymphohistiocytosis Sciencedirect

Interleukin 1 Receptor Antagonist Anakinra In Association With Remdesivir In Severe Covid 19 A Case Report International Journal Of Infectious Diseases
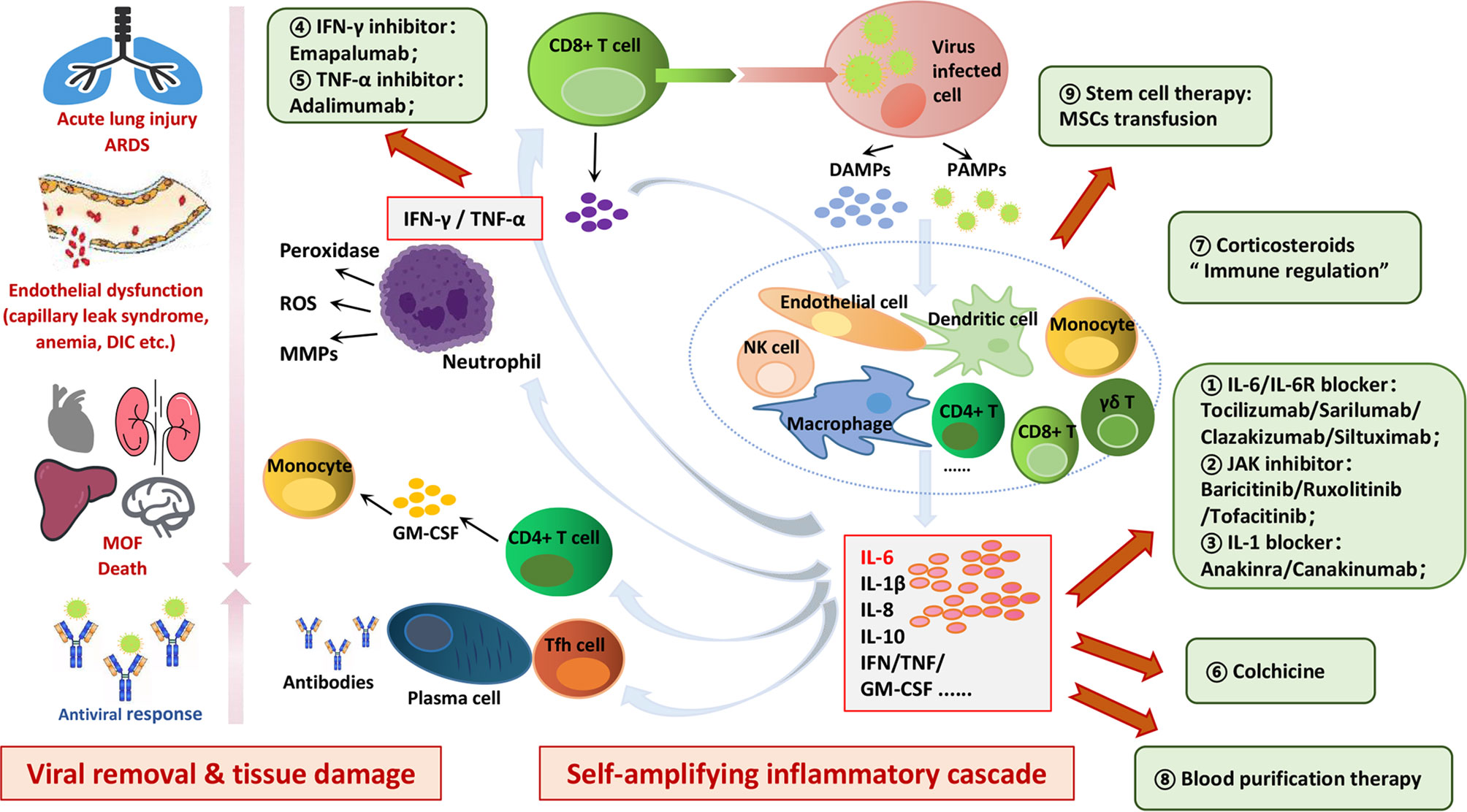
Frontiers Controlling Cytokine Storm Is Vital In Covid 19 Immunology
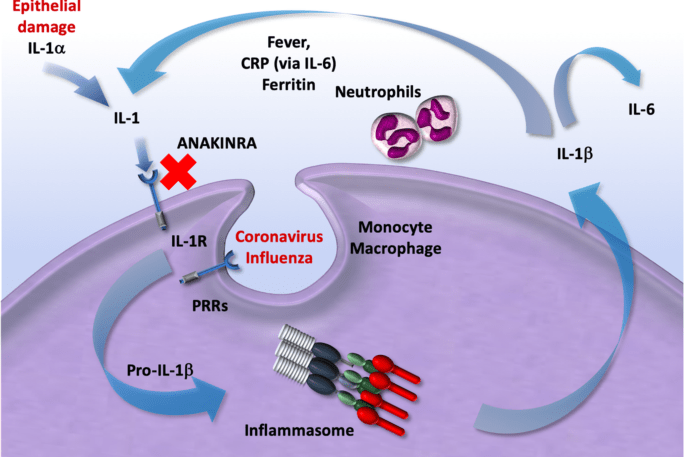
Covid 19 The Role Of Immunomodulators In Treatment

Efficacy Of Early Anti Inflammatory Treatment With High Doses Of Intravenous Anakinra With Or Without Glucocorticoids In Patients With Severe Covid 19 Pneumonia Journal Of Allergy And Clinical Immunology

Il 1 Receptor Antagonist Anakinra In The Treatment Of Covid 19 Acute Respiratory Distress Syndrome A Retrospective Observational Study The Journal Of Immunology

Anakinra Lowers Ventilation Mortality Risk In Non Intubated Patients With Covid 19
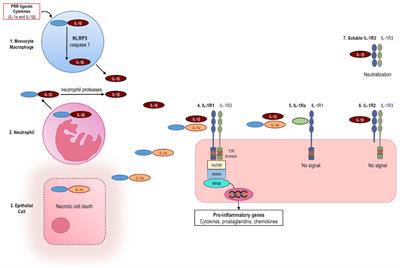
Anakinra List Of Frontiers Open Access Articles

Greek Study Suggests Mortality Benefit For Kineret In Covid 19 2021 05 20 Bioworld

