Anakinra Covid Pediatric : Anakinra Lowers Ventilation Mortality Risk In Non Intubated Patients With Covid 19
Secure gov websites use HTTPS. Some new studies have considered intravenous anakinra for related conditions including hyperinflammation in people with COVID-19 and ARDS.
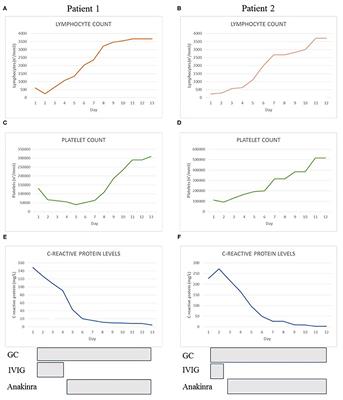
Frontiers Case Report Use Of Anakinra In Multisystem Inflammatory Syndrome During Covid 19 Pandemic Pediatrics
Anakinra a recombinant interleukin IL-1 receptor antagonist may have a beneficial effect in patients with severe COVID-19 with a significant inflammatory response.

Anakinra covid pediatric. Adalimumab certolizumab etanercept golimumab or infliximab. 978-1-4731-3800-1 COVID 19 rapid evidence summary. Pediatric COVID-19 Hospitalizations by State HealthDatagov.
Evidence review commissioned by NHS England. In this cohort study anakinra significantly reduced both need for invasive mechanical ventilation in the ICU and mortality among patients with severe COVID-19 without serious side-effects. They have been admitted to pediatric intensive care unit and have been treated with combination of anakinra 6-8 mgkgdie iv and a standard dose of methylprednisolone 2 mgkgdie in addition to lopinavirritonavir 400 mg q12h and low molecular weight heparin 100 UIkg q12h with good clinical response Conclusion.
We conducted a retrospective cohort study of all SARS-coV2-RNA-positive patients treated with tocilizumab or anakinra in Kaiser Permanente Southern California. Tell the doctor and pharmacist about all of your childs drugs prescription or OTC natural products vitamins and health problems. Severe COVID-19 cases have a detrimental hyper-inflammatory host response and different cytokine-blocking biologic agents were explored to improve outcomes.
Anakinra is effective in reducing clinical signs of hyperinflammation in critically ill COVID-19 patients. However additional trials are needed to better understand the role of IL-1 receptor antagonism in COVID-19 according to a commentary published in Lancet Rheumatology. See the full evidence review for more information.
However its exact benefits for patients with moderate to. Its been fully approved for those. Anakinra blocks the activity of both IL-1α and IL1β and is approved for.
A randomized controlled trial is warranted to draw conclusion about the effects of anakinra. A gov website belongs to an official government organization in the United States. Anakinra could be an effective and safe immunomodulatory treatment to prevent unfavourable outcomes in moderate-to-severe cases of pneumonia due to COVID-19.
Rheumamittel Anakinra erzielt in Beobachtungsstudie klinische Verbesserungen. The https ensures that you are connecting to the official website and that any information you provide is. If your child is taking any of these drugs.
This is not a list of all drugs or health problems that interact with this drug. A pathological dysregulated immune response ie hyperinflammation is a recognised complication of COVID-191 The protype hyperinflammatory syndrome secondary to infection is secondary haemophagocytic lymphohistiocytosis sHLH but the dominant hyperinflammatory phenotype in people with severe COVID-19-associated pneumonia is not that of sHLH and is poorly understood12 The COVID. Treatment with anakinra may reduce mortality risk in patients hospitalized with moderate to severe COVID-19 pneumonia according to findings from a systematic review published in Lancet Rheumatology.
Also these studies do not compare anakinra with other treatments such as tocilizumab. Zur Behandlung der rheumatoiden Arthritis sind in Europa bereits einige Biologika. Additionally anakinra might be helpful in avoiding adverse events such as the secondary infections observed frequently with dexamethasone use and could be considered as an alternative treatment option in specific subgroups eg patients with.
Anakinra use in COVID-19 has been reported in several retrospective and prospective studies as well as in one randomized controlled trial 51415161718192021. Anakinra is administered sc. Anakinra was shown efficacious as first-line monotherapy in single-center prospective cohort trial including 42 pediatric patients median age.
In clinical studies in sepsis doses up to 2 mgkghour iv. Pfizer and BioNTechs Covid vaccine has been authorized for emergency use by the FDA for children 12 to 15 while scientists gather more data on that age group. Dosing was initiated at 2 mgkgdose if after 3 days fever remained the dose was increased to 4 mgkgdose Ter Haar 2019.
Our report demonstrates the occurrence of cytokine storm and hyperinflammation in some children. To examine outcomes among patients who were treated with the targeted anti-cytokine agents anakinra or tocilizumab for COVID-19 -related cytokine storm COVID19-CS. Anakinra for COVID-19 associated secondary haemophagocytic lymphohistiocytosis ES26.
However administering anakinra intravenously is off label which raises safety concerns. Interleukin IL-1 receptor anakinra has been used as an off-label agent for treatment of COVID-19 during the COVID-19 pandemic. Official websites use gov.
At doses of 100 mgday RA or in weight-based doses of up to 8 mgkgday NOMID. So far no specific treatment has been shown to reduce the need for invasive mechanical ventilation and intensive care in patients admitted for COVID-19-associated pneumonia requiring oxygen therapy. Anakinra is effective for cytokine storm syndromes it reportedly works within 2 or 3 days Cron et al.
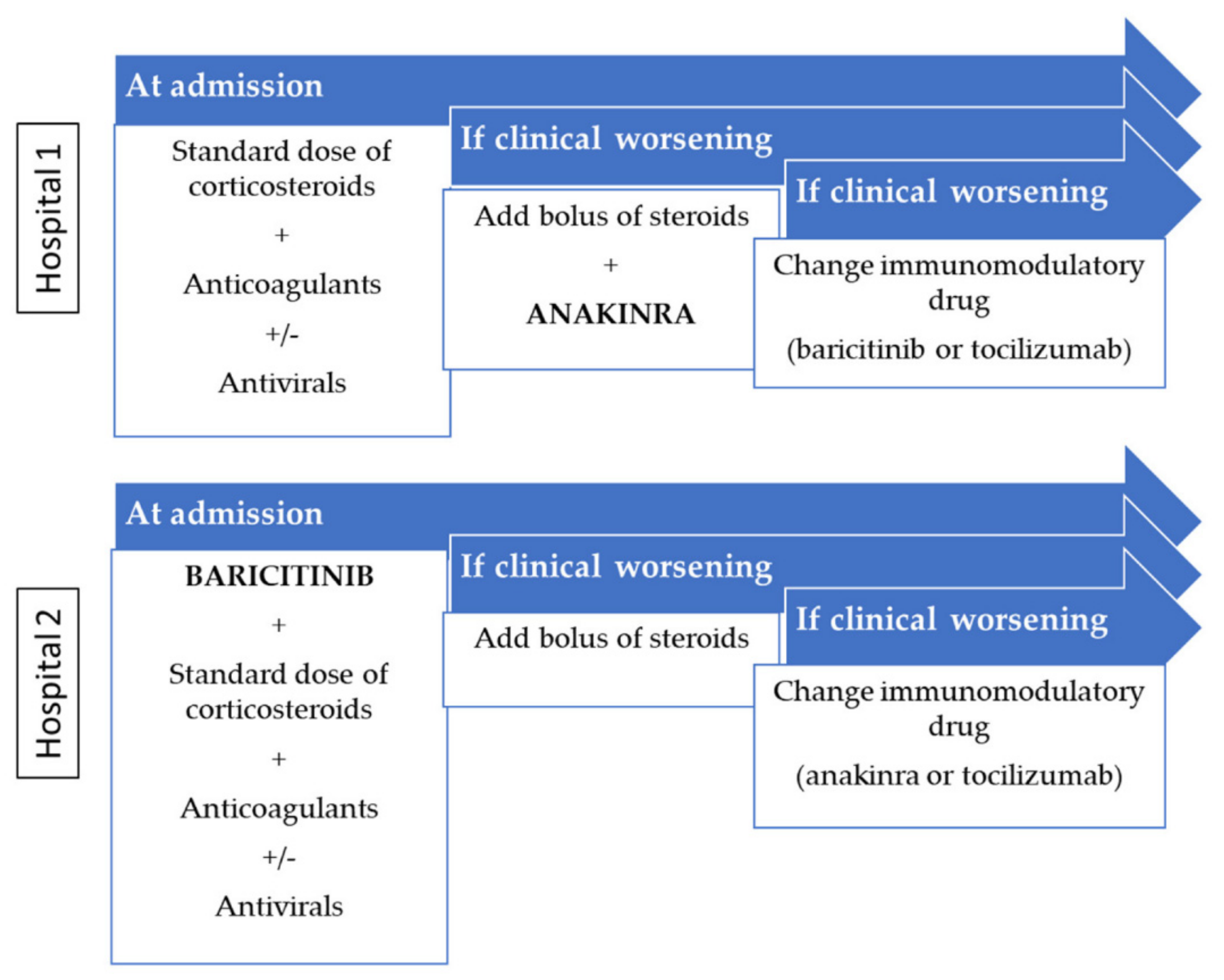
Jcm Free Full Text Anakinra Versus Baricitinib Different Strategies For Patients Hospitalized With Covid 19
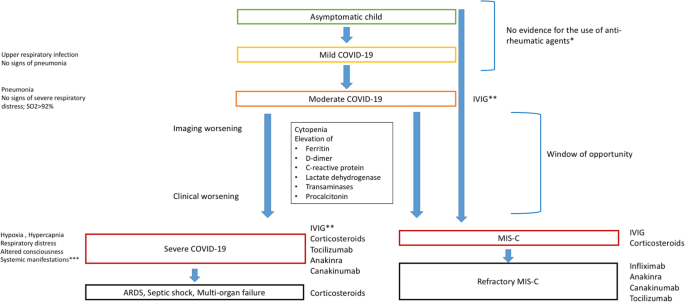
Severe Covid 19 In Pediatric Age An Update On The Role Of The Anti Rheumatic Agents Pediatric Rheumatology Full Text

Favorable Anakinra Responses In Severe Covid 19 Patients With Secondary Hemophagocytic Lymphohistiocytosis Sciencedirect

Anakinra Lowers Ventilation Mortality Risk In Non Intubated Patients With Covid 19

Novel Paediatric Presentation Of Covid 19 With Ards And Cytokine Storm Syndrome Without Respiratory Symptoms The Lancet Rheumatology
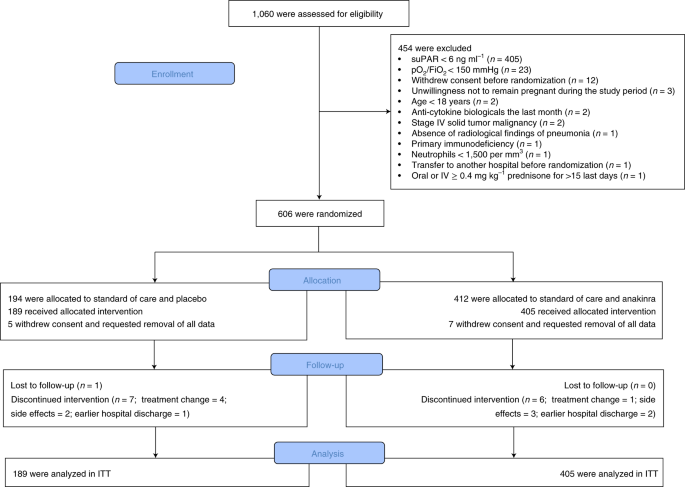
Early Treatment Of Covid 19 With Anakinra Guided By Soluble Urokinase Plasminogen Receptor Plasma Levels A Double Blind Randomized Controlled Phase 3 Trial Nature Medicine

Interleukin 1 Blockade With High Dose Anakinra In Patients With Covid 19 Acute Respiratory Distress Syndrome And Hyperinflammation A Retrospective Cohort Study The Lancet Rheumatology

Safety Perspectives On Presently Considered Drugs For The Treatment Of Covid 19 Penman 2020 British Journal Of Pharmacology Wiley Online Library

Anakinra For Severe Forms Of Covid 19 A Cohort Study The Lancet Rheumatology

Anakinra Reduces Ventilation Need Mortality In Covid 19
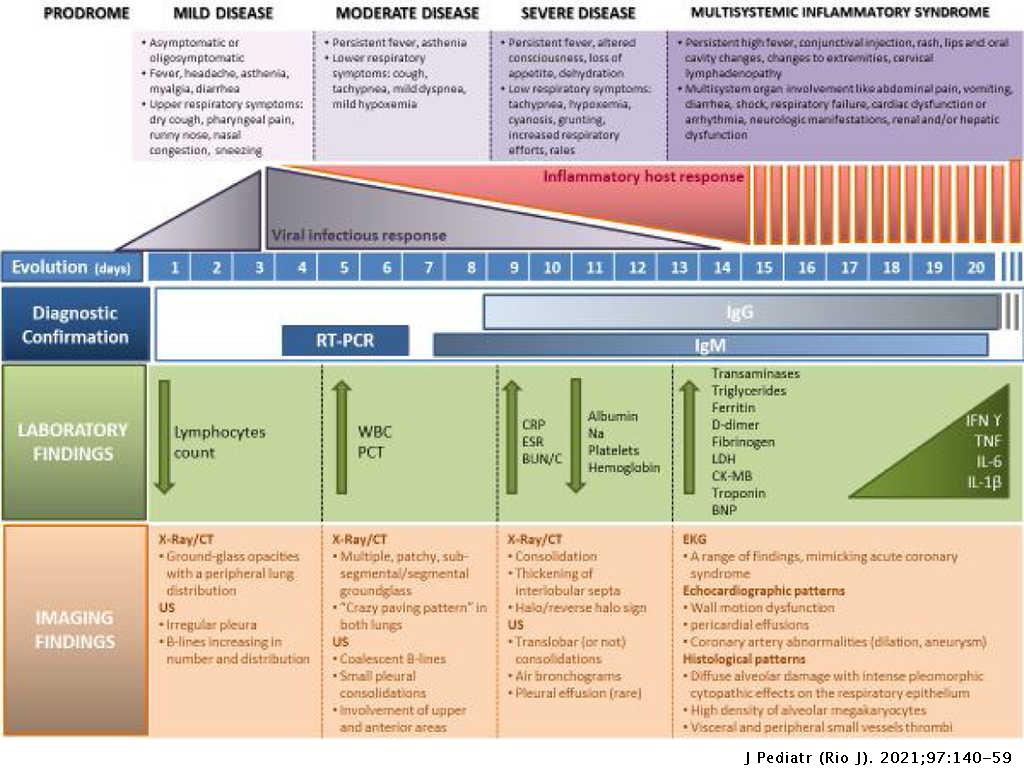
Multisystem Inflammatory Syndrome Associated With Covid 19 From The Pediatric Emergency Physician S Point Of View Jornal De Pediatria

Covid 19 Associated Multisystem Inflammatory Syndrome In Children Mis C Guidelines Revisiting The Western New York Approach As The Pandemic Evolves Sciencedirect

Immunomodulation In Covid 19 The Lancet Respiratory Medicine

Acute Kidney Injury In Pediatric Patients Hospitalized With Acute Covid 19 And Multisystem Inflammatory Syndrome In Children Associated With Covid 19 Kidney International
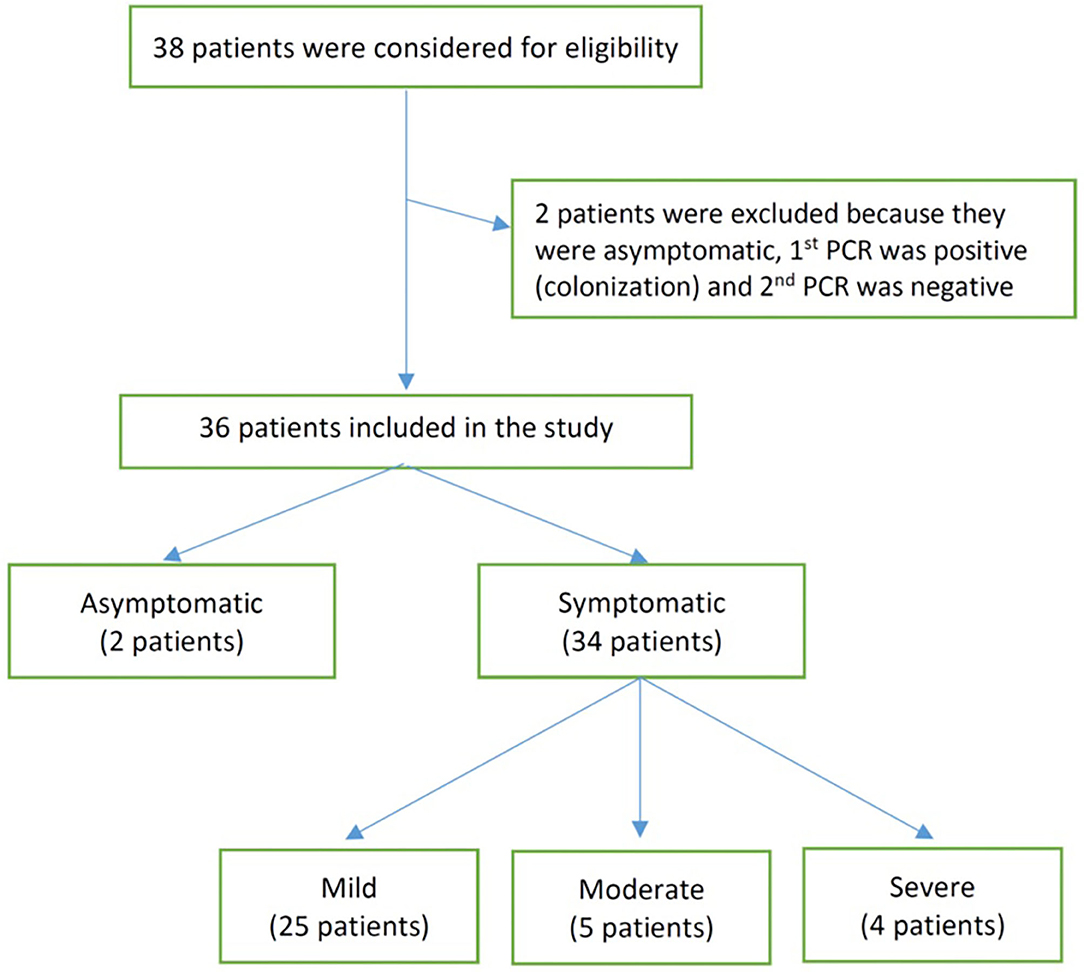
Frontiers Covid 19 Disease In Infants Less Than 90 Days Case Series Pediatrics

Multisystem Inflammatory Syndrome In Children A Systematic Review Eclinicalmedicine

Early Identification Of Covid 19 Cytokine Storm And Treatment With Anakinra Or Tocilizumab International Journal Of Infectious Diseases

Il 1 Receptor Antagonist Anakinra In The Treatment Of Covid 19 Acute Respiratory Distress Syndrome A Retrospective Observational Study The Journal Of Immunology

Anakinra For Patients With Covid 19 A Meta Analysis Of Non Randomized Cohort Studies European Journal Of Internal Medicine