Anakinra Covid Tedavisi : Page 174 Multidisipliner Covid 19
Additionally anakinra might be helpful in avoiding adverse events such as the secondary infections observed frequently with dexamethasone use and could be considered as an alternative treatment option in specific subgroups eg patients with diabetes. Efficient dosage of Anakinra as previously described will be continued until.
Https Toraks Org Tr Site Sf Nmf Pre Migration E65d61774b4874df8502b2e4669167718472471834aeba27abc5ba982836d2db Pdf
İtalyada Mart ayında salgın döneminde bu ilaçla tedavi edilen Covid hastalarında solunum cihazına bağlanma veya ölüm oranının azaldığı görüldü.
Anakinra covid tedavisi. The SAVE-MORE phase 3 study demonstrates the efficacy of anakinra an IL-1αβ inhibitor in patients with COVID-19 and high serum levels of soluble plasminogen activator receptor. Anakinra treatment of COVID-19 patients admitted with LRTI and suPAR concentrations greater or equal than 6 μgl was associated with a relative decrease of the incidence of SRF by 70. This is also.
Ancak bu küçük çaplı ve. A randomized controlled trial is warranted to draw conclusion about the effects of anakinra on clinical outcomes. I tassi di mortalità del Covid-19 sono in buona parte associati allemergere nei pazienti con forme gravi della malattia.
Anakinra is highly effective in MAS and significantly decreased all-cause mortality. At doses of 100 mgday RA or in weight-based doses of up to 8 mgkgday NOMID. EMA has started evaluating an application to extend the use of Kineret anakinra to include treatment of coronavirus disease 2019 COVID-19 in adult patients with pneumonia who are at risk of developing severe respiratory failure inability of the lungs to work properly.
Lanti-infiammatorio Anakinra riduce la mortalità nei pazienti COVID-19 gravi PUBBLICATO IL 05 FEBBRAIO 2021 Uno studio comparativo condotto da Ospedale San Raffaele dimostra la superiorità terapeutica del farmaco anakinra rispetto a tocilizumab e suggerisce limportanza del trattamento precoce. It has been postulated that anakinra a recombinant IL-1 receptor antagonist might help to neutralise the severe acute respiratory syndrome coronavirus 2 SARS-CoV-2-related hyperinflammatory state which is considered to be one cause of acute respiratory distress among patients with COVID-19. We aimed to assess the off-label use of anakinra in patients who were admitted to hospital for severe forms of COVID-19.
Cosè lanakinra lantinfiammatorio che riduce la mortalità del Covid. Treatment with anakinra reduces the need for invasive mechanical ventilation and mortality risk in hospitalized nonintubated patients with COVID-19 according to a meta-analysis published in Rheumatology. Anakinra blocks the activity of.
Dosing of anakinra was determined by clinical experience and the literature that was available at the time. A majority of patients with COVID-19 exhibit a predominant IL-1IL-6 signature but can evolve into an IL-18IFNγ signature mimicking cytokine profiles observed in other inflammatory diseases such as macrophage activation syndrome MAS. Key secondary endpoints included overall mortality.
Over 72 hours were administered to 500 patients and were well tolerated. The total duration of Anakinra is 10 Days. The primary outcomes were severity on an Ordinal Scale measured at day 15 from intervention and days to hospital discharge.
Previous studies have suggested that interleukin IL-1 receptor antagonist anakinra used for the treatment of autoinflammatory disorders may improve hyperinflammatory symptoms in patients. The SAVE-MORE double-blind randomized controlled trial evaluated the efficacy and safety of anakinra an IL-1αβ inhibitor in 594 patients with COVID-19 at risk of progressing to respiratory failure as identified by plasma suPAR 6 ng ml-1 859 n 510 of whom were receiving dexamethasone. Mailand Eine hoch-dosierte intravenöse Behandlung mit dem Interleukin-1-Rezeptor-Antagonisten Anakinra hat in einer Behandlungsserie in Lancet Rheumatology.
Methods Electronic databases were searched on 7 January 2021 to identify studies of immunomodulatory agents anakinra sarilumab siltuximab and tocilizumab for the treatment of COVID-19. Some new studies have considered intravenous anakinra for related conditions including hyperinflammation in people with COVID-19 and ARDS. Kineret is an immunosuppressant a medicine that reduces the activity of.
Severe COVID-19 cases have a detrimental hyper-inflammatory host response and different cytokine-blocking biologic agents were explored to improve outcomes. Anakinra-treated patients who were eventually admitted to the ICU had a shorter stay than those who did not receive anakinra. At the time that our patients were treated ie before the benefit of dexamethasone was shown concern existed about the use of corticosteroids in patients with COVID-19-induced acute respiratory distress syndrome so anakinra was used in preference.
However administering anakinra intravenously is off label which raises safety concerns. Anakinra is effective in reducing clinical signs of hyperinflammation in critically ill COVID-19 patients. Anakinra could be an effective and safe immunomodulatory treatment to prevent unfavourable outcomes in moderate-to-severe cases of pneumonia due to COVID-19.
Results 71 studies totalling 22 058. So apparently the benefit of previous anakinra treatment remained. A prospective cohort study.
In clinical studies in sepsis doses up to 2 mgkghour iv. The patients will receive intraveinous injection of Anakinra 400mg from Day 1 to Day 3 two injections of 100 mg each 12 hours and 200mg the remaining 7 days. At day 28 the adjusted proportional odds of having a worse clinical status assessed by the 11-point World Health Organization.
We thus hypothesized that blocking IL-1 early using anakinra is a therapeutic option in. Anakinra plus Optimized Standard of Care oSOC on the condition of patients with COVID-19 infection and worsening respiratory symptoms. Anakinra is administered sc.
Anakinra treatment in critically ill COVID-19 patients. Also these studies do.
Https Www Ekmud Org Tr Sunum Indir 1358 Panel 3 Immun Modulator Tedaviler Covid 19
Covid Tedavisinde Turk Yogun Bakim Hemsireleri Dernegi Facebook

Anakinra Nedir Covid 19 Tedavisinde Etkili Mi Prof Dr Mustafa Ozdogan
Http Www Ankarabarosu Org Tr Upload Eksayfa Diger Shk 14 04 2020 Lopinavir 200 Mg Ritonavir 50 Mg Film Tablet Pdf
Https Yuksekihtisasuniversitesi Edu Tr Uploads Docs Corona 1591258974 Sars Cov 2 Ile Gelisen Covid 19 Sorular Ve Yanitlari Pdf

Pdf Management Of Immunomodulatory Agents In Covid 19 Treatment
Covid Tedavisinde Turk Yogun Bakim Hemsireleri Dernegi Facebook
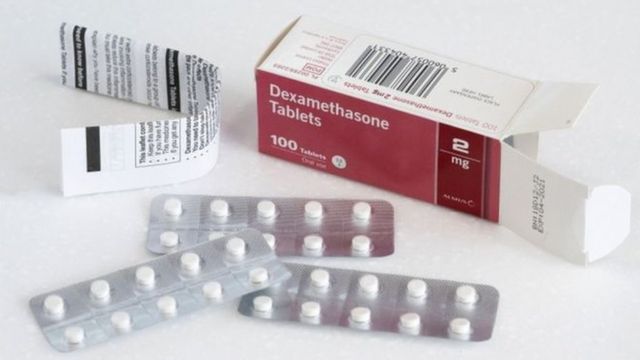
Covid Tedavisi En Basarili Ilaclar Ve Yontemler Hangileri Bbc News Turkce
Covid Tedavisinde Turk Yogun Bakim Hemsireleri Dernegi Facebook
Http Www Ankarabarosu Org Tr Upload Eksayfa Diger Shk 14 04 2020 Lopinavir 200 Mg Ritonavir 50 Mg Film Tablet Pdf

Koronavirus Tedavisi Hangi Asamada Denemeleri Suren Ilaclar Ve Yontemler Neler Independent Turkce
Https Covid19 Tubitak Gov Tr Sites Default Files Inline Files Ahmet Gul Rekombinant Il 1ra Pdf
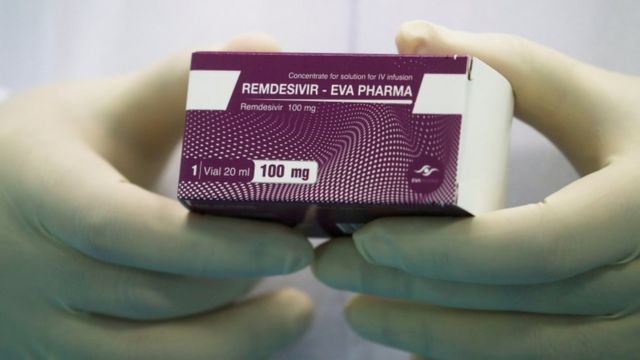
Covid Tedavisi En Basarili Ilaclar Ve Yontemler Hangileri Bbc News Turkce

Page 174 Multidisipliner Covid 19
Https Www Ekmud Org Tr Sunum Indir 1358 Panel 3 Immun Modulator Tedaviler Covid 19

Covid 19 Tedavisinde Kullanilan Ilaclara Gelisen Istenmeyen Ilac Reaksiyonlari Turkiye Ulusal Alerji Ve Klinik Immunoloji Dernegi
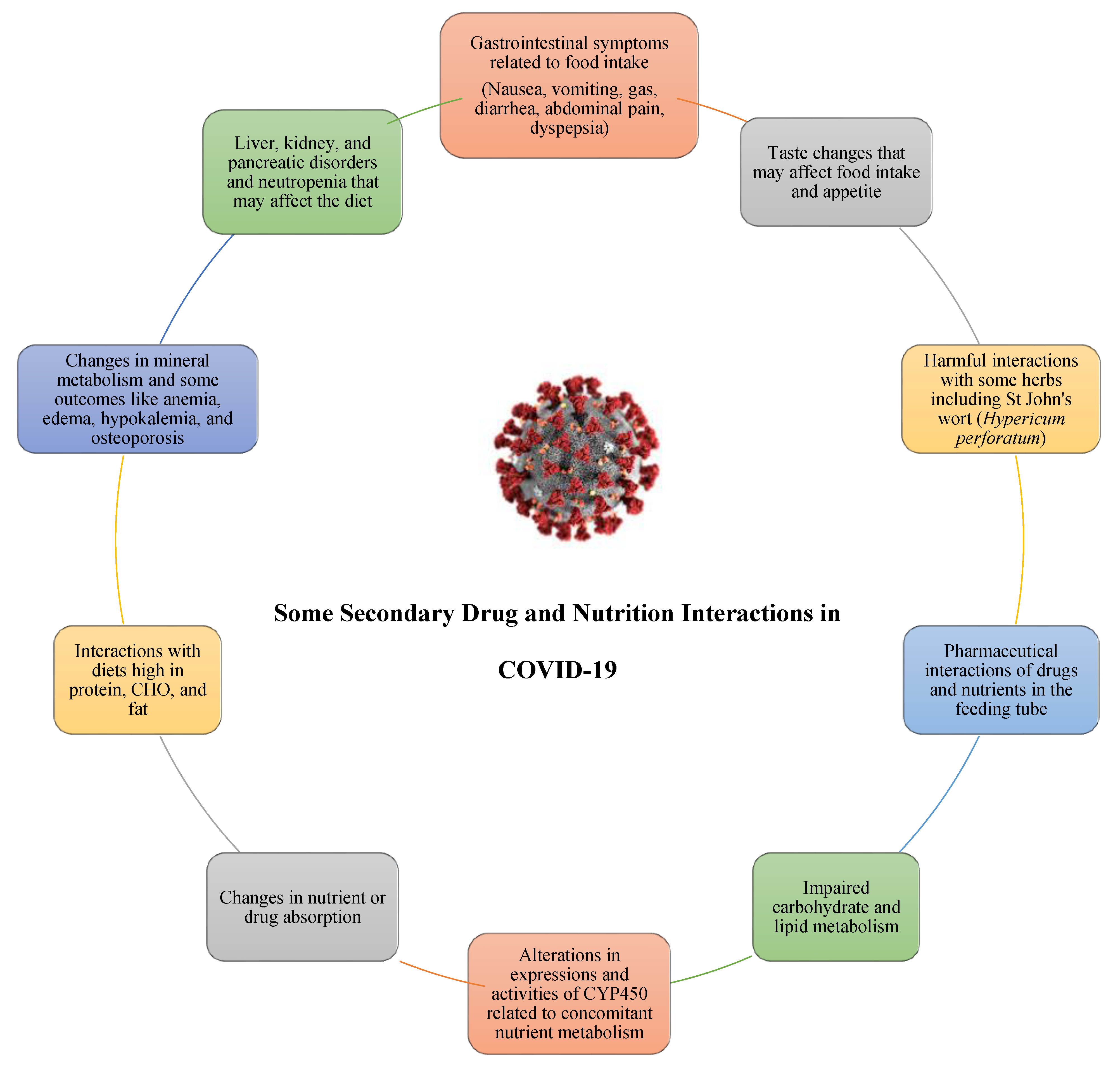
Nutrients Free Full Text Emergent Drug And Nutrition Interactions In Covid 19 A Comprehensive Narrative Review Html


